The chemical reaction in the anode releases electrons which flow as an electric current over the negative pole in the load circuit. The electrons return over the positive pole and are used up in a second chemical reaction inside the cathode by manganese oxide. Power consequently begins to flow on its way from the anode to the cathode, which ultimately causes a flashlight to shine. The more electrons that are available, and the faster they move, the greater the flow of the current will be.
It is difficult to imagine everything that takes place inside such a small battery. Several chemical processes are necessary in the end for a battery to be able to supply power.
How are batteries made?
Batteries are found in any number of everyday appliances, though we rarely give much thought to them. The remote control, the wall clock or toys work all day long - but how are the power bundles inside them actually made? We take a look behind the scenes at the production in Germany. Get a glimpse of the key production steps:
Step 1: The steel container
We produce nearly 1.7 billion batteries a year in various sizes. The first step in production, which is also the basic structure of a conventional alkaline manganese battery, is the steel container that determines battery size (AA, AAA, etc.).
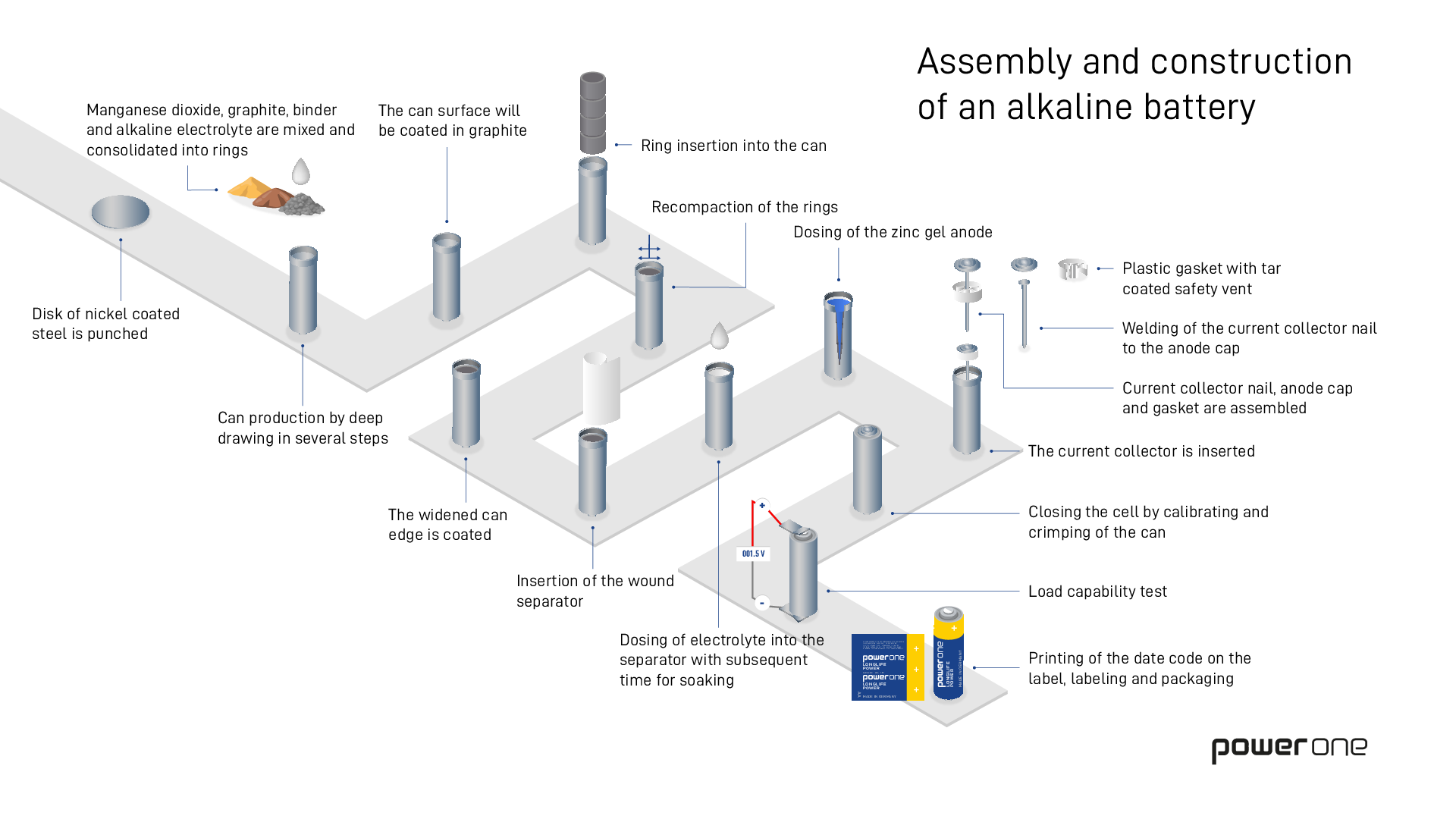
Step 2: Cathode rings
While the container is being shaped, an employee (pictured right) mixes together manganese dioxide (pyrolusite), graphite and electrolyte to form a black granulated material. During the next step of the production process, the granulated material is pressed into silvery matte rings on the cell lines. Four of these rings go into each steel container.

Step 3: The seperator
The open steel containers are placed on ring-shaped conveyors for transportation on the assembly line. A paper strip is rolled into a small tube and sealed on the bottom, used as a separator in the battery which is inserted into the middle of the pressed silvery matte cathode rings.
Step 4: Liquid electrolyte
Step 5: The zinc gel
While the separator is absorbing the liquid electrolyte, zinc powder and potassium hydroxide solution are fed into a mixer. They are mixed for ten minutes to form a light blue paste, the zinc gel. The gel forms the anode of the battery and is then filled into the inner part of the separator.
Step 6: The current collector nail
The stopper is created in a pre-assembly process by soldering the current collector nail to a steel disc and attaching it to the plastic gasket. The steel disc acts as the negative pole. The stopper unit created in this process is then transported to the production line. There it is added into the zinc-gel anode of the filled battery. In order to finally close the battery, the upper edge of the steel can is bent over the stopper unit and is thereby sealing the battery.

Step 7: Packaging

The finished but still naked batteries are stacked on pallets on the production line. Boxes containing up to 850 batteries are stacked on these pallets. The boxes are moved to a conveyor belt by a robot which employees lovingly named “Schorsch”. The belt leads the cells to the packaging process.
In a next step the batteries are given a wrapper and laid into their packaging, so-called blister packs, by a conveyor belt and another robot.
By the time the batteries end up on shelves, and then in our shopping baskets, they have a long journey behind them. Even though batteries are small, a lot of steps are involved in getting them ready for sale.
How does a battery work?
How exactly does a battery actually work? And where does all that power come from?
The inner workings of a battery
A steel container forms the battery casing, which holds the electrodes, an anode (the negative terminal) and a cathode (the positive terminal). The cathode consists of silvery matte rings made of manganese dioxide, graphite and electrolyte. The anode is the zinc paste located inside the separator. The separator keeps the electrodes apart to prevent a short circuit.

What happens between the battery and the electrical device?
What happens in the battery?
Discharge is triggered when a battery is connected to the electrical circuit of an electrical device such as a flashlight. When this happens, the anode and cathode react with one another and an electric charge passes between them. During this process, an ion current flows in the electrolyte and through the separator from the cathode to the anode.



